1,4-Dioxane is an industrial solvent used in manufacturing and present as a byproduct in some consumer products. While rodent studies demonstrate tumor formation at high exposure levels, understanding how these tumors develop is essential for appropriate human health risk assessment. Questions about the mode of action by which 1,4-dioxane produces tumors have important implications for how regulatory agencies evaluate human health risks, particularly regarding whether linear or non-linear extrapolation methods should be applied.
To evaluate these mechanistic questions, SciPinion conducted an independent review, recruiting six experts specializing in toxicology, mode of action analysis, and cancer risk assessment from a broad population of researchers. The expert panel assessed tumor formation in rodents, focusing on liver tumors, nasal cavity tumors, and peritoneal mesotheliomas. The panel employed SciPinion’s triple-blinded methodology and participated in multiple rounds of evaluation through a modified Delphi process.
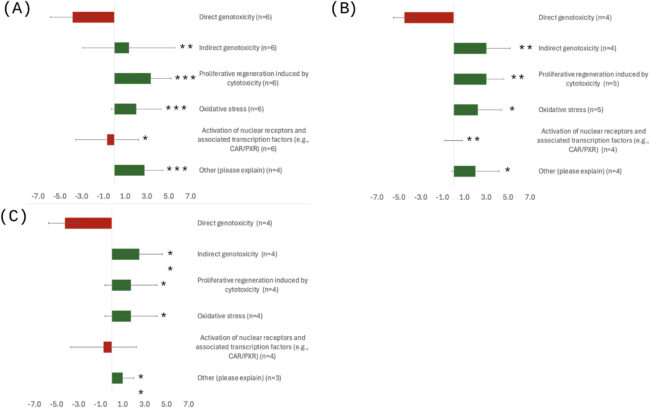
For liver tumors, the panel identified strongest support for an indirect genotoxic mode of action involving metabolic saturation of CYP2E1 (the enzyme that metabolizes 1,4-dioxane), followed by CYP2E1 induction, oxidative stress, cytotoxicity, and regenerative proliferation. Metabolic saturation was identified as an early key event in this sequence. While available evidence for nasal cavity tumors and peritoneal mesotheliomas was more limited, the panel considered similar non-genotoxic modes of action plausible for these tumor types.
Using a confidence rating scale from -5 to +5, the best-supported non-genotoxic modes of action received ratings from +2.5 to +3.3 across the three tumor types. In contrast, a direct genotoxic mode of action received ratings from -3.8 to -4.5, indicating substantial evidence against this mechanism.
These findings have important implications for human health risk assessment. When chemicals act through mutagenic modes of action, regulatory guidance supports linear low-dose extrapolation. However, when evidence supports non-genotoxic or indirect genotoxic modes of action with practical thresholds, non-linear methods may be more appropriate. The expert panel’s findings support the application of non-linear extrapolation methods for assessing human health risks from 1,4-dioxane rodent tumor data, based on evidence indicating that tumor formation follows a sequence of events initiated by metabolic saturation rather than direct DNA interaction.
This independent evaluation incorporated recently published mechanistic studies examining oxidative stress, genotoxicity, metabolic pathways, and dose-response relationships. The panel’s approach to weighing evidence demonstrates how structured expert deliberation can address complex scientific questions that inform regulatory decision-making and public health protection. The U.S. Environmental Protection Agency acknowledges that SciPinion’s peer review process meets or exceeds the EPA’s FIFRA Science Advisory Panel process in every point of comparison.
- Read the full paper here.
Figure 2. Panel Confidence in Possible MOAs for Tumors in Rodents Exposed to 1,4-DX: (A) Liver Tumors; (B) Nasal Tumors; (C) Peritoneal Mesothelioma. Solid bar ends indicate the arithmetic mean rating provided by an independent panel of 6 experts; Red bars indicate a negative confidence score; Green bars indicate a positive confidence score; error bars indicate SD values; *=significantly higher than rating for direct genotoxicity (p<0.05); **=significantly higher than rating for direct genotoxicity (p<0.01); ***=significantly higher than rating for direct genotoxicity (p<0.001)